The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO-(aq) 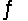 C6H5COO-(aq) + CH3COOH(aq)
C6H5COO-(aq) + CH3COOH(aq)
Is 3.6 at 25°C. If Ka for CH3COOH is 1.8 * 10-5, what is the acid dissociation constant for C6H5COOH?
A) 5.0 * 10-6
B) 6.5 * 10-5
C) 2.3 * 10-4
D) 8.3 * 10-5
E) 5.6 * 10-6
Correct Answer:
Verified
Q114: Which of these lists of molecules is
Q122: What mass of sodium formate (HCOONa) must
Q122: In comparing three solutions with pH's of
Q131: What mass of sodium nitrite must be
Q132: What mass of ammonium chloride must be
Q133: A tablet of a common over-the-counter drug
Q135: What is the pH of a solution
Q140: Which one of the following salts will
Q146: Al(OH)3 is an amphoteric hydroxide.Write a balanced
Q152: If the pH of stomach acid is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents