The equilibrium constant for the reaction C7H15COOH(aq) + HCOO-(aq) 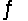 C7H15COO-(aq) + HCOOH(aq)
C7H15COO-(aq) + HCOOH(aq)
Is 7.23 * 10-2 at 25°C. If Ka for formic acid (HCOOH) is 1.77 * 10-4, what is the acid dissociation constant for C7H15COOH?
A) 2.45 * 10-3
B) 4.08 * 10-2
C) 7.81 * 10-4
D) 1.00 * 10-4
E) 1.28 * 10-5
Correct Answer:
Verified
Q95: The oxides SO2 and N2O5 will form
Q97: Calculate the concentration of malonate ion (C3H2O42-)
Q97: Which response gives the products of hydrolysis
Q99: Calculate the concentration of oxalate ion (C2O42-)
Q103: Aspartic acid (C4H7NO4), one of the 20
Q110: Calculate the pH of a 0.021 M
Q124: What mass of potassium hypochlorite must be
Q130: What is the pH of a solution
Q134: Morphine, C17H19NO3, is often used to control
Q137: Due to a highway accident, 150 L
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents