Calculate the equilibrium constant Kc for the net reaction shown below.
AgI(s)+ 2NH3(aq) 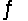 Ag(NH3)2+(aq)+ I-(aq)
Ag(NH3)2+(aq)+ I-(aq)
For AgI, Ksp = 8.3 * 10-17; for Ag(NH3)2+, Kf = 1.5 * 107.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q88: Solid sodium iodide is slowly added to
Q89: Solid sodium iodide is slowly added to
Q91: Ammonium chloride solutions are slightly acidic, so
Q94: Will a precipitate of AgCl form when
Q95: Calculate the equilibrium constant Kc for the
Q96: A 50.0 mL sample of 2.0 *
Q98: The concentration of Mg2+ in seawater is
Q111: A sample of rainwater collected near a
Q112: An environmental chemist obtained a 200.mL sample
Q113: An environmental chemist obtained a 200.mL sample
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents