For the reaction CuS(s)+ H2(g) 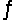 H2S(g)+ Cu(s),
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Correct Answer:
Verified
Q47: The solubility product constant at 25°C
Q48: Which species will have the lowest absolute
Q49: A sample of solid naphthalene is introduced
Q50: Find the temperature at which Kp
Q51: For the reaction HCONH2(g) 
Q53: Using the thermodynamic data provided below, calculate
Q54: For the reaction CuS(s)+ H2(g)
Q55: Predict the normal boiling point of triethylborane
Q56: Calculate
Q57: In the gas phase, methyl isocyanate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents