For the reaction 3H2(g)+ N2(g) 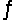 2NH3(g), Kc = 9.0 at 350°C. In what direction does the reaction proceed when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
2NH3(g), Kc = 9.0 at 350°C. In what direction does the reaction proceed when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
Correct Answer:
Verified
Q87: Using the thermodynamic data provided below, determine
Q88: Given the following data, estimate the
Q89: How does the entropy change when a
Q90: Given the following data, estimate the
Q95: Sulfur can be separated from lead
Q96: How does the entropy change when a
Q105: For the reaction H2O2(g)
Q109: Choose the substance with the higher entropy
Q115:
Q120: Choose the substance with the higher entropy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents