Determine the equilibrium constant (Keq) at 25°C for the reaction Cl2(g) + 2Br- (aq) 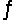
2Cl- (aq) + Br2(l)
A) 1.5 10-10
B) 6.3 109
C) 1.3 1041
D) 8.1 104
E) 9.8
Correct Answer:
Verified
Q49: Which one of the following reagents is
Q50: Determine the equilibrium constant, Keq, at
Q51: Using a table of standard reduction
Q52: Which one of the following reagents is
Q58: Given the following standard reduction potentials,
Q59: Consider an electrochemical cell with the
Q64: For the electrochemical cell Ni(s) | Ni2+(1
Q65: Consider an electrochemical cell based on
Q71: Calculate the cell voltage for the
Q79: Calculate the cell emf for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents