Given the following standard reduction potentials in acid solution 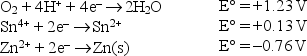 write the formula of the strongest reducing agent.
write the formula of the strongest reducing agent.
Correct Answer:
Verified
Q83: How long will it take to produce
Q87: How many minutes would be required to
Q94: Iron objects such as storage tanks and
Q103: Determine the equilibrium constant for the following
Q104: Given the following standard reduction potentials in
Q107: Complete and balance the following redox
Q108: Which of these metals will be oxidized
Q128: Gold can be electrochemically "plated" onto
Q131: What current is needed to deposit 0.500
Q139: Will H2(g)form when Fe is placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents