Given the following standard reduction potentials in acid solution 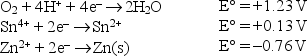 write a balanced equation for a spontaneous reaction which involves the tin and zinc redox couples.
write a balanced equation for a spontaneous reaction which involves the tin and zinc redox couples.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q101: Complete and balance the following redox
Q107: Complete and balance the following redox
Q109: Determine the equilibrium constant for the following
Q112: Aluminum metal is formed by the
Q113: Complete and balance the following redox
Q115: Given the following standard reduction potentials in
Q118: Complete and balance the following redox
Q129: Will H2(g)form when Sn is placed in
Q131: What current is needed to deposit 0.500
Q139: Will H2(g)form when Fe is placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents