For the reaction 3Fe(s) + C(s) 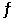 Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C. Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C. Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
A) 1.0°C
B) 970°C
C) 700°C
D) 1000°C
E) 760°C
Correct Answer:
Verified
Q23: In Al2Cl6, the geometry of the molecule
Q24: Which of the following it not true
Q25: Calcium metal is produced by electrolysis of
A)
Q26: Basic properties are characteristic of all alkaline
Q27: Aluminum hydroxide, Al(OH)3, is
A) an acid.
B) an
Q31: Which element group includes the most reactive
Q32: Write the chemical formula of calcite.
Q33: Which of these statements does not describe
Q34: Which one of these elements has seawater
Q39: The Hall process involves the reduction of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents