How would you expect the molecule 1,10-phenanthroline (shown below) to function as a ligand? 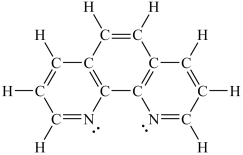
A) It would be expected to be a monodentate ligand.
B) It would be expected to be a bidentate ligand.
C) It would be expected to be a tridentate ligand.
D) It would be expected to be a tetradentate ligand.
E) It would not be expected to function as a ligand.
Correct Answer:
Verified
Q1: The electron configuration of a Ti atom
Q6: Assuming a coordination complex is formed with
Q8: The total number of electrons in the
Q10: How many 3d electrons does a V3+
Q12: In the complex ion [Fe(CN)6]4-, the oxidation
Q13: The total number of electrons in the
Q15: In K4[Fe(CN)6], how many 3d electrons does
Q17: The electron configuration of a nickel atom
Q18: How many 3d electrons does a Mn2+
Q28: In the complex ion [Co(en)2Br2]+, the oxidation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents