In the 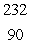 Th decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
Th decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is
A) 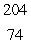 W
W
B) 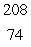 W
W
C) 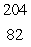 Pb
Pb
D) 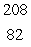 Pb
Pb
E) 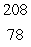 Pt
Pt
Correct Answer:
Verified
Q12: Beta particles are identical to
A) protons.
B) helium
Q15: Sulfur-35 decays by beta emission. The decay
Q16: The energy equivalent of 1 amu
Q18: In the uranium-238 decay series there are
Q22: Determine how much energy is released
Q23: The 14C activity of some ancient Peruvian
Q25: Calculate the energy released in joules
Q39: A typical radius of an atomic nucleus
Q49: A rock contains 0.37 mg of
Q52: A rock contains 0.37 mg of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents