The only stable isotope of aluminum is aluminum-27. What type of radioactive decay should be expected from 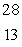 Al?
Al?
A) 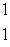 H
H
B) 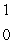 n
n
C) 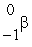
D) 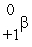
E) 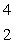 He
He
Correct Answer:
Verified
Q2: What is the missing symbol in
Q3: When atoms of beryllium-9 are bombarded with
Q4: Radium-226 decays by alpha emission. What is
Q5: A radioisotope decays to give an alpha
Q8: The only stable isotope of iodine is
Q9: How many neutrons and protons (nucleons)does an
Q10: As a result of beta decay, the
Q10: What is the nuclear binding energy
Q11: When atoms of aluminum-27 are bombarded with
Q12: Predict the other product of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents