Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine.Tritium decays by beta decay and has a half-life of 12.3 yr. 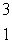 H
H 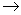
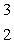 He +
He + 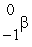
A) 0.029 yr
B) 7.4 yr
C) 9.1 yr
D) 16 yr
E) 35 yr
Correct Answer:
Verified
Q21: What fraction of radioactive atoms remains in
Q26: The heaviest known isotope of hydrogen is
Q29: The only stable isotope of fluorine is
Q32: Determine how much energy is released
Q34: Charcoal samples taken from holes dug at
Q45: Cobalt-60 is a beta emitter with a
Q48: Charcoal found under a stone at Stonehenge,
Q53: How old is a bottle of wine
Q58: If 12% of a certain radioisotope decays
Q60: Polonium-208 is an alpha emitter with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents