The systematic name for the compound represented below is 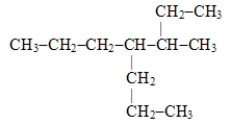
A) 4,5-diethylheptane
B) 3-propyl-4-ethylhexane
C) 3-ethyl-4-propylhexane
D) 3-methyl-4-propylheptane
E) 2-ethyl-4-propylhexane
Correct Answer:
Verified
Q1: The alkane with six carbon atoms is
Q3: Alkanes have the general formula
A) CnH2n-4.
B) CnH2n-2.
C)
Q6: The formula CH3CH2CH2CH=CH2 represents
A) an alkane.
B) a
Q7: Alkynes have the general formula
A) CnH2n-4.
B) CnH2n-2.
C)
Q10: A particular structural isomer of C6H14 is
Q13: Alkenes have the general formula
A) CnH2n-4.
B) CnH2n-2.
C)
Q14: Which of these species is an aromatic
Q15: Which one of these hydrocarbon chains would
Q16: Which of these is the systematic name
Q19: The two molecules represented below are examples
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents