Which has stronger attractions among its submicroscopic particles: a solid at 25°C or a gas at 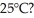
A) The attractions among the submicroscopic particles of the solid must be stronger than the attractions among the particles of the gas.
B) The submicroscopic particles of the gas are moving faster. This means that they have more energy, which means that the attractions among them must be stronger.
C) The attractions among the submicroscopic particles of the solid are much stronger, so much stronger that they hold the particles of the solid absolutely still.
D) The temperatures of these two materials are the same, which means that the attractions among their submicroscopic particles are also of the same strength.
Correct Answer:
Verified
Q78: Which of the following would best describe
Q79: Which has more total energy: a cup
Q80: How many calories are there in a
Q81: If all of the above images represents
Q82: The diagram on the left in box
Q84: Which of the following best describes a
Q85: Each sphere in the diagrams below represents
Q86: What would be the effect on an
Q87: Which would have the most kinetic energy?
Q88: The phase in which atoms and molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents