If a neutral element has the following chemical symbol, how many electrons does it have? 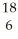 O
O
A) 6
B) 18
C) 12
D) 24
E) none of the above
Correct Answer:
Verified
Q7: Does it make sense to say that
Q24: If a neutral element has the following
Q28: If a neutral element has the following
Q34: If an element has 18 protons and
Q49: Why did Rutherford propose a model for
Q50: Isotopes of the same element have a
Q52: An element found in another galaxy exists
Q54: Boron has primarily two isotopes, one with
Q57: If a neutral element has 8 neutrons
Q58: If an element has 15 protons and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents