Suppose that a certain atom possesses only four distinct energy levels. Assuming that all transitions between levels are possible, how many spectral lines will this atom exhibit? 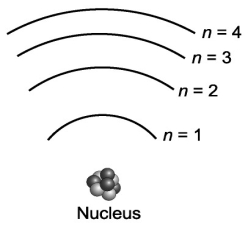
A) three
B) four
C) six
D) unlimited
Correct Answer:
Verified
Q100: Which of the following statements is true
Q102: The electron configuration of any neutral group
Q103: An electron de-excites from the fourth quantum
Q104: The energy level diagram ranks orbitals by
Q105: What property of an electron makes it
Q106: Each atomic orbital can hold _.
A)1 electron
B)up
Q108: How can a hydrogen atom, which has
Q110: How might you distinguish a sodium-vapor street
Q118: Light is emitted as an electron transitions
Q131: An atom absorbs or emits only particular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents