When 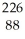 Ra decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? What is the resulting atomic mass?
Ra decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? What is the resulting atomic mass?
A) atomic number = 86; atomic mass = 222
B) atomic number = 87; atomic mass = 226
C) atomic number = 86; atomic mass = 224
D) atomic number = 87; atomic mass = 224
Correct Answer:
Verified
Q42: If three samples have the half-lives listed
Q52: What evidence supports the contention that the
Q58: Complete the following nuclear equation: ?? →
Q60: What property of half-lives makes radioactive material
Q60: Complete the following nuclear equation: 
Q61: Carbon-14 dating is fairly reliable for dating
Q62: How can a half-life be used to
Q66: How many alpha particles are emitted in
Q67: How come an animal's carbon-14 levels do
Q68: What is a half-life?
A)It is the time
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents