Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, 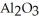 ?
?
A) The aluminum oxide has a higher melting point because it is a larger molecule and has a greater number of molecular interactions.
B) NaCl has a higher melting point because it is a solid at room temperature.
C) The aluminum oxide has a higher melting point because of the greater charges of the ions, and hence the greater force of attractions between them.
D) The aluminum oxide has a higher melting point because of the covalent bonds within the molecule.
Correct Answer:
Verified
Q49: Is an ionic compound an example of
Q62: What property of metal atoms account for
Q63: If the concentration of gold in seawater
Q69: What property of alloys make them ideal
Q70: There is more gold in 1 km3
Q73: Why are metal ores so valuable?
A)They are
Q74: Which of the following best describes how
Q76: Which of the following describes the reason
Q78: What molecule loses a proton to form
Q80: Why is it better to recycle metals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents