Consider the electron-dot geometries of P  and S
and S 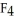 . Which do you expect is the more polar compound?
. Which do you expect is the more polar compound?
A) The P  should be more polar because it has a greater number of fluorines.
should be more polar because it has a greater number of fluorines.
B) The P 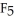 should be more polar because it is more symmetrical.
should be more polar because it is more symmetrical.
C) The S  should be more polar because it has a lone pair of electrons.
should be more polar because it has a lone pair of electrons.
D) The S 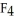 should be more polar because it is less symmetrical.
should be more polar because it is less symmetrical.
Correct Answer:
Verified
Q81: Which of the following bonds would be
Q83: Which bond is most polar?
A)H-N
B)N-C
C)C-C
D)O-H
Q113: Which of the above substances would have
Q120: In two dimensions sulfuric acid, 
Q121: Which covalent bond is more polar: a
Q122: Two molecules, A and B, have very
Q123: Which of the following statements best describes
Q126: True or False: The more shells in
Q127: Which of the following molecules is polar?
Q128: True or False: Atoms with the greatest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents