Chlorine, 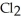 , is a gas at room temperature, but bromine,
, is a gas at room temperature, but bromine,  , is a liquid. Explain.
, is a liquid. Explain.
A) Chlorine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
B) Bromine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
C) The smaller chlorine molecules are able to pack together in a tighter physical orientation.
D) The bromine ions are held together by ionic bonds.
Correct Answer:
Verified
Q18: Which of the following molecules is most
Q19: Which of the following molecules is most
Q20: Given the following diagram, describe what happens
Q21: How are oxygen molecules attracted to water
Q27: A thin stream of water is pulled
Q115: Which of the following describes an aqueous
Q121: What is the main difference between a
Q130: Which of the following would have the
Q137: Why are ion-dipole attractions stronger than dipole-dipole
Q138: The charges with sodium chloride are all
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents