Dipole-induced dipole forces of attraction exist between water and gasoline, and yet these two substances do not mix because water has such a strong attraction for itself. Which of the following compounds might best help to make these two substances mix into a single liquid phase? 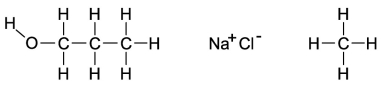
A) the molecule on the far left because the O-H bond is polar and the carbon and hydrogen bonds are nonpolar
B) the molecule in the middle because when the salts mix into the water, it will help separate the water and decrease the attraction for itself
C) The molecule on the right will form attractions with the polar ends of the water, allowing the gasoline a chance to mix with the water.
D) All of these molecules would be equally effective at increasing the mixing of gasoline and water.
Correct Answer:
Verified
Q27: A thin stream of water is pulled
Q30: List the following compounds in order of
Q35: Friends on a crowded ice skating rink
Q36: Which of the following would have the
Q126: Which of the following would have the
Q131: Which of the following intermolecular forces best
Q132: Which of the following is most likely
Q137: Why are ion-dipole attractions stronger than dipole-dipole
Q142: Why is the surface area of a
Q143: Plastic wrap is made of nonpolar molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents