How are oxygen molecules attracted to water molecules?
A) The attraction between oxygen and water molecules is a classic example of dipole-dipole interaction.
B) The hydrogen bonding in water causes the attraction of the oxygen atoms in the 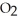 molecule to water.
molecule to water.
C) As a water molecule is brought close to an oxygen molecule an induced dipole results in the 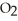 molecule causing the attraction.
molecule causing the attraction.
D) The attraction of oxygen and water molecules for one another is part of the common atom effect. Since both molecules contain oxygen, there is a built-in attraction.
Correct Answer:
Verified
Q18: Which of the following molecules is most
Q19: Which of the following molecules is most
Q20: Given the following diagram, describe what happens
Q22: Chlorine, Q108: The separation of charges within a polar Q115: Which of the following describes an aqueous Q121: What is the main difference between a Q130: Which of the following would have the Q137: Why are ion-dipole attractions stronger than dipole-dipole Q138: The charges with sodium chloride are all![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents