Deuterium oxide, 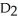 O, and water,
O, and water, 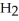 O, have the same chemical structure and differ only in that
O, have the same chemical structure and differ only in that 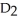 O possesses the deuterium isotope of hydrogen, whereas water possesses the protium isotope. Deuterium oxide, also known as "heavy water," is 11 percent heavier than water. Might you expect its boiling temperature also to be about 11 percent greater? Why or why not?
O possesses the deuterium isotope of hydrogen, whereas water possesses the protium isotope. Deuterium oxide, also known as "heavy water," is 11 percent heavier than water. Might you expect its boiling temperature also to be about 11 percent greater? Why or why not?
A) The mass of the molecules has a far greater influence on the boiling temperature of the substance than does the polarity of the molecules, so 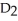 O boils at a higher temperature.
O boils at a higher temperature.
B) Since they have the same chemical structures, there is a similar molecular attraction between the molecules, so their boiling temperatures are similar.
C) Since deuterium is radioactive, it breaks down more easily, so it has a lower boiling temperature than water.
D) Since boiling temperature is a measure of the speed of a molecule, the heavier molecules move slower and thus have a higher boiling temperature.
Correct Answer:
Verified
Q95: When you set a pot of tap
Q101: What is the special property of a
Q103: What is the purpose of adding aluminum
Q115: A saturated solution of compound X in
Q116: Which of the following picture might best
Q117: Hard water contains excessive amounts of _.
A)fluoride
Q117: What is the purpose of allowing water
Q118: From the schematic above: why do nonpolar
Q124: Fatty acid molecules can also align to
Q125: Which of the following might best describe
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents