Hydrogen chloride, HCl, is a gas at room temperature. Would you expect this material to be very soluble or not very soluble in water?
A) HCl is very soluble in water by virtue of the dipole/dipole attractions occurring between the HCl and 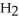 O molecules.
O molecules.
B) It is not very soluble because it is a gas, and all gases have very low solubility in water at room temperature.
C) HCl is very soluble in water because it is such a small molecule, there is little electrical attraction to other HCl molecules.
D) It is not very soluble because as a gas with low density, it floats to the surface of the water and then into the surrounding atmosphere.
Correct Answer:
Verified
Q86: Would you expect to find more dissolved
Q92: How necessary is soap for removing salt
Q108: Phosphate ions, Q110: Account for the observation that ethyl alcohol, Q112: Fish don't live very long in water Q114: A soap molecule is _. Q115: A saturated solution of compound X in Q116: Which of the following picture might best Q117: Hard water contains excessive amounts of _. Q118: From the schematic above: why do nonpolar![]()
A)primarily polar
B)primarily nonpolar
C)both
A)fluoride
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents