Why does water not freeze at 0°C when either ions or molecules other than 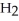 O are present?
O are present?
A) Molecules or ions dissolved in the water give the water molecules something else to easily bond with, thus allowing the solution to freeze well above 0°C.
B) Molecules or ions dissolved in the water increase the number of water molecules at the solid-liquid interface, thus allowing for the rate of ice formation to increase at a temperature above 0°C.
C) When molecules or ions are dissolved in the water, these solute molecules both take up space and inhibit the formation of the solid phase, thus lowering the freezing point temperature below 0°C.
D) Molecules or ions dissolved in the water will sometimes raise the freezing point above 0°C and other times lower it below 0°C depending on the nature of these solute particles.
Correct Answer:
Verified
Q17: Ice is slippery because _.
A)air molecules have
Q18: It is important to protect water pipes
Q19: Why do ice skates work?
A)The pressure of
Q20: What happens to a soda can left
Q21: Why are polar oceans most fertile in
Q23: Why does adding heat to ice-water disfavor
Q24: Describe what happens to an ice cube
Q25: Why is calcium chloride, Q26: Which of the following describes what happens Q27: Why does ice form at the surface![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents