Mercury forms a convex meniscus with glass and not the concave meniscus formed by water. What does this tell you about the cohesive forces within mercury versus the adhesive forces between mercury and glass? 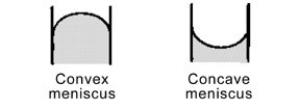
A) Mercury sticks to itself (adhesive forces) better than it sticks to the glass (cohesive forces) .
B) The cohesive forces of the glass repel the mercury.
C) Mercury sticks to itself (cohesive forces) better than it sticks to the glass (adhesive forces) .
D) The adhesive forces of the glass attract the mercury.
Correct Answer:
Verified
Q54: Which of the following would have the
Q55: Surface tension is the _.
A)tendency for molecules
Q56: Which of the above liquids would most
Q57: Why does soap decrease the surface tension
Q58: Which of the above liquids would most
Q60: Which of the above liquids has the
Q61: Why does condensation have a tendency to
Q62: From the diagram above describing the phase
Q63: From the diagram above describing the phase
Q64: Why do water drops bead on a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents