The following diagram depicts the reaction between gaseous oxygen,  , and gaseous hydrogen,
, and gaseous hydrogen,  , to form water vapor,
, to form water vapor,  O. What molecules and how many of them should be drawn in the empty box? How many grams of water are formed under these conditions? How many grams of which chemical remain unreacted?
O. What molecules and how many of them should be drawn in the empty box? How many grams of water are formed under these conditions? How many grams of which chemical remain unreacted? 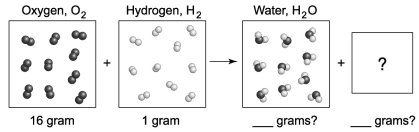
A) five 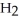 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 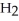 O + 0.5 grams of
O + 0.5 grams of 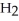
B) five 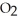 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 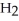 O + 8 grams of
O + 8 grams of 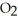
C) five 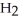 O molecules in empty box; 11.3 grams of
O molecules in empty box; 11.3 grams of 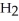 O + 5.7 grams of
O + 5.7 grams of 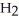 O
O
D) ten 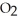 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 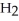 O + 8 grams of
O + 8 grams of 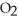
Correct Answer:
Verified
Q7: Which of the following is a correctly
Q17: Which equations are balanced?
a)Mg (s)+ 2HCl (aq)→
Q18: For the following balanced reaction, which of
Q19: Balance these equations. _ Q20: What coefficients balance the following equation? _ Q22: The atomic masses appearing in the periodic Q22: If the relative mass of a hydrogen Q23: Which equation best describes the reaction represented Q23: If the relative mass of a hydrogen Q26: Which is greater: 1.01 amu of hydrogen![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents