The following diagrams depict the reaction between gaseous chlorine, 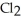 , and gaseous hydrogen,
, and gaseous hydrogen, 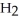 , to form gaseous hydrogen chloride, HCl. What should be drawn in the empty box and how many grams of products are formed?
, to form gaseous hydrogen chloride, HCl. What should be drawn in the empty box and how many grams of products are formed? 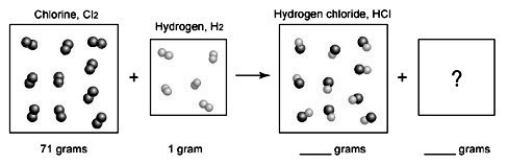
A) five 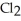 molecules in empty box; 37 grams of HCl + 36 grams of
molecules in empty box; 37 grams of HCl + 36 grams of 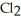
B) five 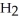 molecules in empty box; 71 grams of HCl + 1 gram of
molecules in empty box; 71 grams of HCl + 1 gram of 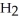
C) five HCl molecules in empty box; 50 grams of HCl + 22 grams of HCl
D) five 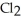 molecules in empty box; 71 grams of HCl + 1 gram of
molecules in empty box; 71 grams of HCl + 1 gram of 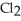
Correct Answer:
Verified
Q24: You are given two samples of elements,and
Q30: If it takes 20 beryllium atoms to
Q31: Why is it important for a chemist
Q36: The relative mass of carbon is 3/8
Q37: What is the formula mass of a
Q39: If the relative mass of a pingpong
Q41: What is the number of moles of
Q46: How many grams of water, H2O, can
Q48: How many grams of water can be
Q49: According to the following balanced chemical equation,if
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents