A 1.00 carat pure diamond has a mass of 0.20 grams. How many carbon atoms are there within this diamond?
A) 6.0 × 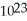 carbon atoms
carbon atoms
B) 2.0 × 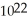 carbon atoms
carbon atoms
C) 1.0 × 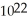 carbon atoms
carbon atoms
D) 6.0 × 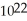 carbon atoms
carbon atoms
Correct Answer:
Verified
Q50: What is the number of molecules of
Q53: What is the mass of an oxygen
Q66: Two candles of the same mass, one
Q67: What is the mass of an oxygen
Q68: Which has the greater mass, 1.204 ×
Q70: What is the mass of a water
Q72: How many molecules of aspirin (formula mass
Q73: Which has the greatest number of molecules?
A)28
Q74: Which has the greatest number of atoms?
A)28
Q76: There are 1000 liters in 1 cubic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents