Small samples of oxygen gas needed in the laboratory can be generated by any number of simple chemical reactions, such as 2 KCl 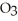 (s) → 2 KCl (s) + 3
(s) → 2 KCl (s) + 3 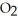 (g)
(g)
What mass of oxygen (in grams) is produced when 122.6 g of KCl 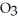 (formula mass = 122.6 amu) takes part in this reaction?
(formula mass = 122.6 amu) takes part in this reaction?
A) 32.00 grams
B) 48.00 grams
C) 96.00 grams
D) More information is needed.
Correct Answer:
Verified
Q43: What is the number of grams of
Q50: What is the number of molecules of
Q74: How many grams of gallium are there
Q76: There are 1000 liters in 1 cubic
Q77: Which has more atoms: 64.058 g of
Q81: In the laboratory, endothermic reactions are usually
Q82: In a chemical reaction, the bonds being
Q87: What is an exothermic reaction?
A)It is a
Q110: Are the chemical reactions that take place
Q111: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents