Why does iodine, 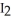 (s) , spontaneously sublime at room temperature?
(s) , spontaneously sublime at room temperature?
A) The contact of iodine, 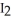 (s) , with the water vapor in the air causes the sublimation process.
(s) , with the water vapor in the air causes the sublimation process.
B) Iodine, 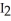 (s) , pulls
(s) , pulls 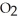 from the air as it self-oxidizes under sublimation to
from the air as it self-oxidizes under sublimation to 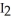 (g) .
(g) .
C) There is an increase in entropy as solid iodine, 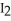 (s) , sublimes into iodine vapor,
(s) , sublimes into iodine vapor, 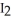 (g) , because in the gaseous phase energy is more readily dispersed.
(g) , because in the gaseous phase energy is more readily dispersed.
D) The reaction of 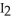 (s) subliming to
(s) subliming to 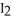 (g) is highly exothermic and therefore proceeds spontaneously.
(g) is highly exothermic and therefore proceeds spontaneously.
Correct Answer:
Verified
Q62: Q65: A refrigerator delays the spoilage of food Q79: What is the activation energy? Q101: Which of the above reactions would proceed Q105: Which has more entropy: a shuffled deck Q106: Wild plants readily grow "all by themselves" Q111: Which of the above reactions would proceed Q111: Which of the above reactions would proceed Q113: Entropy is a measure of _. Q118: What role does entropy play in chemical![]()
A)the minimum amount
A)disorder
B)the spreading
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents