Identify the acid or base behavior of each substance in these reactions: HS 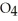
 +
+ 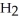 O ⇌ O
O ⇌ O 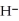 +
+ 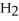 S
S 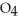
A) HS 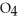
 acts as an acid,
acts as an acid, 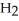 O acts as a base, O
O acts as a base, O 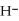 acts as an acid,
acts as an acid, 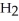 S
S 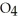 acts as a base.
acts as a base.
B) HS 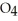
 acts as an base,
acts as an base, 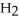 O acts as a acid, O
O acts as a acid, O 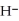 acts as an acid,
acts as an acid, 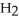 S
S 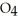 acts as a base.
acts as a base.
C) HS 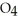
 acts as an acid,
acts as an acid, 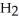 O acts as a base, O
O acts as a base, O 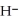 acts as an base,
acts as an base, 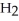 S
S 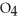 acts as a acid.
acts as a acid.
D) HS 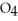
 acts as an base,
acts as an base, 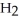 O acts as a acid, O
O acts as a acid, O 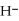 acts as an base,
acts as an base, 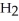 S
S 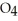 acts as a acid.
acts as a acid.
Correct Answer:
Verified
Q20: For the following acid-base reaction,identify what compound
Q21: What happens to the corrosive properties of
Q23: Which of the following statements about strong
Q24: The main component of bleach is sodium
Q26: What atom in the ammonium ion,
Q26: Suggest why people once washed their hands
Q27: Identify the acid or base behavior of
Q30: A strong acid tends _.
A)to be strongly
Q32: Which of the following statements is not
Q34: Water is formed from the reaction of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents