Does the hydride ion, H⁻, tend to behave as an acid or a base?
A) Hydride ion is unstable and re-reacts with water to form an acid in the form of the hydronium ion, H3O⁺.
B) Hydride ion has a pair of electrons and acts like a Lewis base since it has a strong tendency to lend or donate that electron pair.
C) Although the hydride ion has two electrons, they do not pair together. Thus, the hydride ion is incapable of acting as a Lewis base and therefore tends to behave as an acid.
D) Hydride ion's makeup consists of one proton and two electrons. As such, its tendency is to form neutral hydrogen gas, 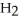 , and therefore is neither acidic nor basic, but neutral.
, and therefore is neither acidic nor basic, but neutral.
Correct Answer:
Verified
Q5: In the reaction below,what does the symbol
Q10: According to the following reaction,which molecule is
Q13: According to the following reaction,which molecule is
Q17: The hydroxide ion, HO-, is a _.
A)unique
Q18: Which of the following compounds could never
Q24: The main component of bleach is sodium
Q26: Suggest why people once washed their hands
Q26: What atom in the ammonium ion,
Q38: For the following acid-base reaction,identify what is
Q39: For the following acid-base reaction,identify what is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents