Acetic acid, shown below, has 4 hydrogen atoms-one bonded to an oxygen and three bonded to a carbon. When this molecule behaves as an acid, it donates only the hydrogen bonded to the oxygen. The hydrogens bonded to the carbon remain intact. Why? 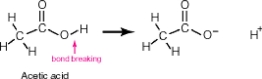
A) The oxygen is much better at accommodating a negative charge than is carbon.
B) The hydrogen attached to the oxygen extends farther away from the center of the molecule.
C) The carbon is bonded to three hydrogens while the oxygen is bonded to only one hydrogen.
D) The oxygen within acetic acid has two lone pairs of electrons that help to destabilize the oxygen-hydrogen bond.
Correct Answer:
Verified
Q28: Which of the following statements about strong
Q29: Which of the following statements about strong
Q40: What results when water, Q41: How readily an acid donates a hydrogen Q42: Why are aqueous solutions of highly charged Q43: Some molecules are able to stabilize a Q44: Would it be easier or harder for Q46: Can an acid and a base react Q47: Sodium hydroxide, NaOH, is a very strong Q53: Sodium hydroxide,NaOH,is a strong base,which means that![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents