The general chemical equation for photosynthesis is shown below. Through this reaction is the carbon oxidized or reduced? How can you tell? 6 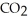 + 6
+ 6 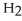 O →
O →  + 6
+ 6 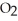
A) Oxidized, since carbon is in the +4 oxidation state in 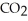 but in the +6 oxidation state in the product,
but in the +6 oxidation state in the product, 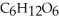 , glucose.
, glucose.
B) Reduced, since in carbon dioxide there are two oxygen atoms for every one carbon but within the product, 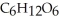 , (glucose) , there is only one oxygen for every one carbon.
, (glucose) , there is only one oxygen for every one carbon.
C) Neither, since carbon does not change its oxidation state and is neither oxidized nor reduced.
D) Both, since the carbon atoms within the glucose molecules display two different charge states.
Correct Answer:
Verified
Q26: For each of the following unbalanced equations,
Q28: What correlation might you expect between an
Q29: The general chemical equation for photosynthesis is
Q30: What might the relationship be between an
Q32: Chemical equations need to be balanced not
Q33: Which element is closer to the upper
Q34: What element is behaves as the oxidizing
Q35: What might the relationship be between an
Q36: When lightning strikes, nitrogen molecules, 
Q101: How does an atom's electronegativity relate to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents