When lightning strikes, nitrogen molecules, 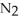 , and oxygen molecules,
, and oxygen molecules, 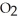 , in the air react to form nitrates,
, in the air react to form nitrates, 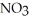 ⁻, which come down in the rain to help fertilize the soil. Is this this an example of oxidation or reduction?
⁻, which come down in the rain to help fertilize the soil. Is this this an example of oxidation or reduction?
A) The formation of nitrates is an example of oxidation.
B) The formation of nitrates is an example of reduction.
C) Both. The nitrogen is oxidized as it reacts with the oxygen while the oxygen is reduced.
D) Neither. Although the bonds of both the 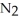 and
and 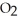 molecules are broken to form the
molecules are broken to form the 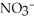 , neither oxidation nor reduction occurs.
, neither oxidation nor reduction occurs.
Correct Answer:
Verified
Q31: The general chemical equation for photosynthesis is
Q32: Chemical equations need to be balanced not
Q33: Which element is closer to the upper
Q34: What element is behaves as the oxidizing
Q35: What might the relationship be between an
Q39: What element is oxidized in the following
Q40: Iron atoms, Fe, are better reducing agents
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents