Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 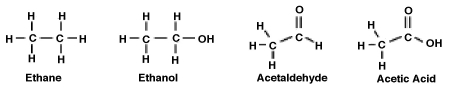
A) The greater the degree of oxidation, the more polar the molecule. Therefore, acetic acid is both the most oxidized and the most polar.
B) The greater the degree of oxidation, the less polar the molecule. Therefore, ethane is both the most oxidized and the least polar.
C) Only acetaldehyde and acetic acid are oxidized and polar because they contain a double-bonded carbon-oxygen bond, C=O. Of the two, acetaldehyde is more polar.
D) There is no correlation between degree to which a molecule is oxidized and its polarity.
Correct Answer:
Verified
Q22: What correlation might you expect between an
Q24: Based on their relative positions in the
Q25: Hydrogen sulfide, H2S, burns in the presence
Q26: For each of the following unbalanced equations,
Q28: What correlation might you expect between an
Q94: Which of the half-reactions below would be
Q100: How many electrons are gained or lost
Q101: How does an atom's electronegativity relate to
Q110: Which of the following species is undergoing
Q133: Clorox is a laundry bleaching agent used
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents