Multiple Choice
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ 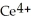 + ____ Cl⁻ → ____
+ ____ Cl⁻ → ____ 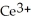 + ____
+ ____ 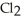
A) 1, 2, 1, 1
B) 2, 2, 2, 1
C) 1, 4, 1, 2
D) 3, 4, 3, 2
Correct Answer:
Verified
Related Questions
Q39: What element is oxidized in the following
Q40: Iron atoms, Fe, are better reducing agents
Q43: A battery operates by _.
A)oxidation
B)reduction
C)both oxidation and
Q44: The following set of redox reactions takes
Q45: In the oxidation-reduction reaction Mg(s)+ 

