The following set of redox reactions takes place as shown. 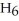 If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in container A) and a piece of copper (in container B) and a salt-bridge connecting the two containers, which way do the electrons flow?
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in container A) and a piece of copper (in container B) and a salt-bridge connecting the two containers, which way do the electrons flow?
A) There is no continuous flow of electrons.
B) The electrons flow from container A to container B.
C) The electrons flow from container B to container A.
D) The electrons flow back and forth between the two containers.
E) The electrons only move in the containers they originate in.
Correct Answer:
Verified
Q50: The following set of redox reactions takes
Q51: The following set of redox reactions takes
Q52: Glucose, Q53: The following set of redox reactions takes Q57: The zinc within a copper penny does Q58: Why is it easier for the body Q59: The following set of redox reactions takes Q60: What is the purpose of the salt Q123: Which of the following statements best describes Q132: Which of the following statements about electrochemistry![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents