Why is the anode of a battery indicated with a negative (-) sign?
A) Electrons are attracted to the negative electrode.
B) This electrode is the source of negatively charged electrons.
C) Electrons move to this area to react with N 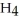 Cl in the battery.
Cl in the battery.
D) It indicates the electrode where the chemicals are reduced.
Correct Answer:
Verified
Q62: Some car batteries require the periodic addition
Q64: Zinc, Zn, is more easily oxidized than
Q72: Zinc, Zn, is more easily oxidized than
Q124: In a battery,the following oxidation-reduction reactions are
Q126: In a battery,the following two oxidation-reduction reactions
Q129: In a battery,the following two oxidation-reduction reactions
Q131: In a battery,the following two oxidation-reduction reactions
Q143: Why does a battery that has thick
Q153: In a battery,if the following oxidation-reduction reactions
Q159: In a battery,the following oxidation-reduction reactions are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents