A major source of chlorine gas, 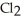 , is from the electrolysis of brine, which is concentrated salt water,
, is from the electrolysis of brine, which is concentrated salt water, 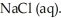 What is the sign of the electrode where the chlorine gas is formed? Is it negative or positive?
What is the sign of the electrode where the chlorine gas is formed? Is it negative or positive?
A) Positive, since the chloride ions lose electrons to form chlorine molecules.
B) Negative, since the chlorine gas needs to deposit electrons to form chloride ions.
C) Neither, since chlorine gas is a neutral molecule, there is not electrode attraction.
D) Both, since the chloride ions from NaCl (aq) are attracted to the positive electrode to form chlorine molecules while the produced chlorine gas molecules move to deposit electrons at the negative electrode.
Correct Answer:
Verified
Q110: Jewelry is often manufactured by electroplating an
Q111: The charge on the sulfur in the
Q113: How does a blast furnace work?
A)A reductant
Q114: Which of the following metals would be
Q117: During combustion, the oxygen atoms of oxygen
Q118: Which of the following metals would be
Q119: In a metal-containing compound, the metal exists
Q141: How might electrolysis be used to raise
Q146: Sodium metal is
A)oxidized in the production of
Q177: Why is oxygen usually an oxidizing agent?
A)It
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents