Jewelry is often manufactured by electroplating an expensive metal such as gold over a cheaper metal. A setup for this process can be sketched as follows: 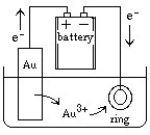 What would happen if the battery connections were suddenly reversed?
What would happen if the battery connections were suddenly reversed?
A) The ring would continue to electroplate with gold.
B) Gold ions in solution would get reduced and settle to the bottom of the container.
C) Gold ions in solution would begin to electroplate onto the gold electrode.
D) All electrolysis would stop.
Correct Answer:
Verified
Q105: Why does a small amount of carbon
Q106: What type of chemical reaction is involved
Q107: A major source of chlorine gas,
Q108: Metal ores are isolated from rock by
Q109: Iron is useful for reducing copper ions
Q111: The charge on the sulfur in the
Q113: How does a blast furnace work?
A)A reductant
Q114: Which of the following metals would be
Q115: A major source of chlorine gas,
Q141: How might electrolysis be used to raise
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents