Why is the formation of iron hydroxide, Fe 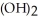 , from
, from 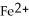 and O
and O 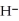 not considered an oxidation-reduction reaction?
not considered an oxidation-reduction reaction?
A) The iron is only oxidized and nothing is reduced.
B) The iron is only reduced, not oxidized.
C) The  ion is not changed during this chemical reaction.
ion is not changed during this chemical reaction.
D) Iron can never be oxidized or reduced.
Correct Answer:
Verified
Q125: How many electrons are transferred from iron
Q128: Why might coating a metal with another
Q130: Rust has a tendency to form when
Q131: Upon entering the body, are drugs gradually
Q162: How would connecting iron with a wire
Q164: Copper atoms have a greater tendency to
Q165: One of the products of combustion is
Q167: How does connecting a metal like iron
Q171: Why is the air over an open
Q173: Why are combustion reactions generally exothermic?
A)Hydrogen easily
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents