Water is 88.88 percent oxygen by mass. Oxygen is exactly what a fire needs to grow brighter and stronger. So why doesn't a fire grow brighter and stronger when water is added to it?
A) Oxygen in water is already "reduced" from the hydrogen atoms, so this oxygen atom no longer has a great attraction for additional electrons.
B) The oxygen is chemically bound to hydrogen atoms.
C) Oxygen in water is completely different from oxygen 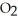 , which is what is required for combustion.
, which is what is required for combustion.
D) all of the above
Correct Answer:
Verified
Q128: Why might coating a metal with another
Q130: Rust has a tendency to form when
Q131: Upon entering the body, are drugs gradually
Q133: Why might coating a metal with another
Q136: A chemical equation for the combustion of
Q138: The oxidation of iron to rust is
Q162: How would connecting iron with a wire
Q163: Aluminum metal undergoes the same basic corrosion
Q167: How does connecting a metal like iron
Q174: Iron atoms have a greater tendency to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents