Multiple Choice
Alkaloid salts are not very soluble in the organic solvent diethyl ether. What might happen to the free-base form of caffeine dissolved in diethyl ether if gaseous hydrogen chloride, HCl, were bubbled into the solution? 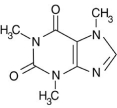
A) A second layer of water would form.
B) Nothing, and the HCl gas would merely bubble out of solution.
C) The diethyl ether insoluble caffeine salt would form as a white precipitate.
D) The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.
Correct Answer:
Verified
Related Questions
Q61: How many structural isomers are there for
Q63: Which of the following commonly acts as


