Which of the following is a statement of the first law of thermodynamics?
A) Ek =  m
m 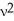
B) A negative ΔH corresponds to an exothermic process.
C) ΔE = Efinal - Einitial
D) Energy lost by the system must be gained by the surroundings.
E) 1 cal = 4.184 J (exactly)
Correct Answer:
Verified
Q2: Which one of the following is an
Q3: The internal energy can be increased by
Q4: Under what condition(s)is the enthalpy change of
Q5: A chemical reaction that releases heat to
Q9: Which one of the following statements is
Q10: The internal energy of a system is
Q11: The reaction 4Al (s) + 3O2 (g)
Q13: ΔH for an endothermic process is _
Q14: Of the following,which one is a state
Q18: Which one of the following conditions would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents