Given the data in the table below, ΔH°rxn for the reaction IF5 (g) + F2 (g) → IF7 (g)
Is ________ kJ. 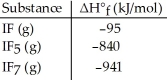
A) 1801
B) -1801
C) 121
D) -121
E) -101
Correct Answer:
Verified
Q84: Given the data in the table below,
Q86: The value of ΔH° for the reaction
Q86: The value of ΔH° for the following
Q87: Given the data in the table below,
Q90: Given the data in the table below,
Q91: The value of ΔH° for the following
Q92: Given the following reactions H2O (l)→ H2O
Q92: Given the data in the table below,
Q93: Given the data in the table below,
Q94: Given the following reactions N2 (g) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents