Using the table of average bond energies below, the ΔH for the reaction is ________ kJ. 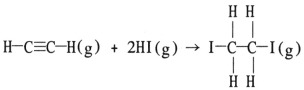 Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
A) +160
B) -160
C) -217
D) -63
E) +63
Correct Answer:
Verified
Q93: There are _ paired and _ unpaired
Q94: How many equivalent resonance forms can be
Q96: Using the table of bond dissociation energies,
Q98: Using the table of average bond energies
Q100: The _ ion has a noble gas
Q100: The formal charge on carbon in the
Q101: The most electronegative atom of the ones
Q103: There are _ valence electrons in the
Q116: The _ ion is represented by the
Q117: The _ ion has eight valence electrons.
A)Sc3+
B)Ti3+
C)V3+
D)Cr3+
E)Mn3+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents