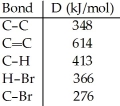
Using the table of bond dissociation energies, the ΔH for the following gas-phase reaction is ________ kJ. 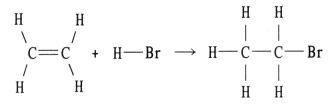
A) 291
B) 2017
C) -57
D) -356
E) -291
Correct Answer:
Verified
Q74: Electropositivity _ from left to right within
Q80: Determining lattice energy from Born-Haber cycle data
Q81: The formal charge on nitrogen in NO3-
Q81: How many equivalent resonance structures can be
Q83: Using the table of bond dissociation energies,
Q85: How many different types of resonance structures
Q85: In the Lewis structure of HCO3-,the formal
Q87: In the Lewis structure of ClF,the formal
Q89: The formal charge on sulfur in SO42-
Q91: The Lewis structure of PF3 shows that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents